Electrolysis of brine
Posted by Midnyt Blaze on 21:06 with No comments
Electrolysis of brine :
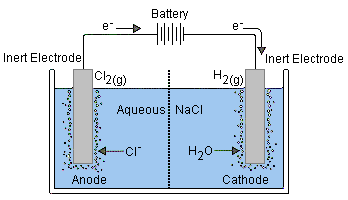
Electrolysis of brine gives hydrogen gas at the cathode and chlorine gas at the anode. Sodium hydroxide remains dissolved in the solution.
The half equations are :-
 |
| Electrolysis of brine |
At the cathode: Hydrogen ions gain electrons (reduction) to form hydrogen atoms. The hydrogen atoms combine to form molecules of hydrogen gas.
2H+ + 2e- 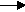 H2
H2
At the anode: Chloride ions lose electrons (oxidation) to form chlorine atoms. The chlorine atoms combine to form molecules of chlorine gas.
2Cl- - 2e- 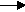 Cl2
Cl2
The overall reaction is
2NaCl(aq) + 2H2O(l) 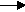 2Na+(aq) + 2OH-(aq) + Cl2(g)+ H2(g)
2Na+(aq) + 2OH-(aq) + Cl2(g)+ H2(g)
Categories: Electrochemistry









0 comments:
Post a Comment