Ideal gas
Ideal gas
An ideal gas is a gas whose pressure P, volume V, and temperature T are related by the ideal gas law
At normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many gases such as nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances. Generally, a gas behaves more like an ideal gas at higher temperature and lower pressure, as the work which is against intermolecular forces becomes less significant compared with the particles' kinetic energy, and the size of the molecules becomes less significant compared to the empty space between them.
PV = nRT .
where:
P is the pressure of the gas
V is the volume of the gas
n is the amount of substance of gas (also known as number of moles)
R is the ideal, or universal, gas constant, equal to the product of the Boltzmann constant and the Avogadro constant = 8.3145 J/mol K.
T is the temperature of the gas
P is the pressure of the gas
V is the volume of the gas
n is the amount of substance of gas (also known as number of moles)
R is the ideal, or universal, gas constant, equal to the product of the Boltzmann constant and the Avogadro constant = 8.3145 J/mol K.
T is the temperature of the gas







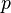 is the pressure,
is the pressure, is the normal force,
is the normal force, is the area of the surface on contact.
is the area of the surface on contact.







