Boyle's law
Posted by Midnyt Blaze on 10:12 with No comments
For a fixed mass of gas at constant temperature, the volume is inversely proportional to the pressure.
Mathematically, Boyle's law can be stated as
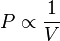
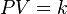
where P is the pressure of the gas, V is the volume of the gas, and k is a constant.
The equation states that product of pressure and volume is a constant for a given mass of confined gas as long as the temperature is constant. The law can be usefully expressed as


Categories: Laws, Thermodynamics









0 comments:
Post a Comment