Charle's Law
Posted by Midnyt Blaze on 10:37 with No comments
For a fixed mass of gas at constant pressure, the volume is directly proportional to the kelvin temperature.
this directly proportional relationship can be written as:


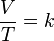
where:
- V is the volume of the gas
- T is the temperature of the gas (measured in Kelvin).
- k is a constant.
This law describes how a gas expands as the temperature increases; conversely, a decrease in temperature will lead to a decrease in volume. For comparing the same substance under two different sets of conditions, the law can be written as:
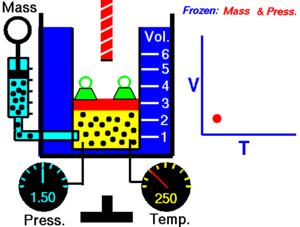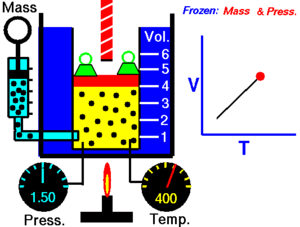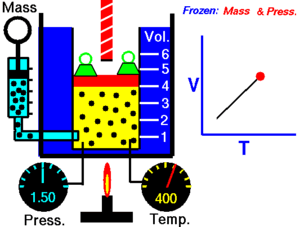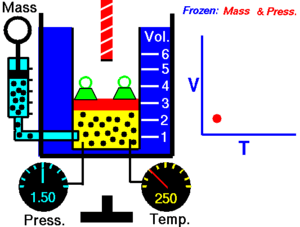 |
| An animation demonstrating the relationship between volume and temperature. |
Categories: Laws, Thermodynamics









0 comments:
Post a Comment